Bryce Hanna
@photobiogenesis
THE DEFINITIVE GUIDE TO MAGNESIUM Today we're going back to the basics with an overview of my favorite mineral of all time, magnesium, one of the cornerstones of life on earth Why magnesium is so important, and how to use it to improve your health THREAD //
This thread will be split into two sections: The chemistry of magnesium, and how to supplement magnesium If you're only interested in practical advice feel free to scroll down to the second section Now without further ado, let's jump right in
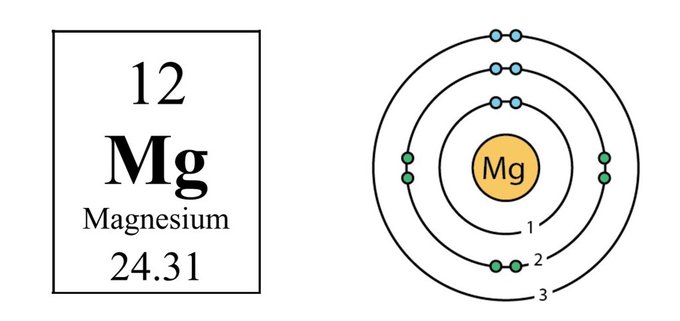
MAGNESIUM CHEMISTRY Magnesium is what's known as a divalent cation This means it has two outer orbital electrons, and it tends to lose them giving it a +2 charge in solution It's found in group 2 in the periodic table with other divalent cations like calcium and beryllium
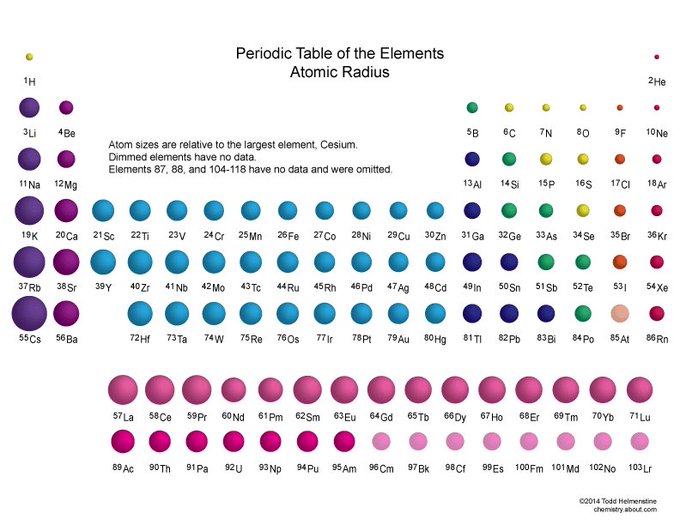
The defining property of magnesium as an element is its high charge/size ratio Compared to the other cations used in biology (calcium, sodium, and potassium) magnesium has the smallest atomic radius but +2 charge This plays a role in how magnesium interacts with water
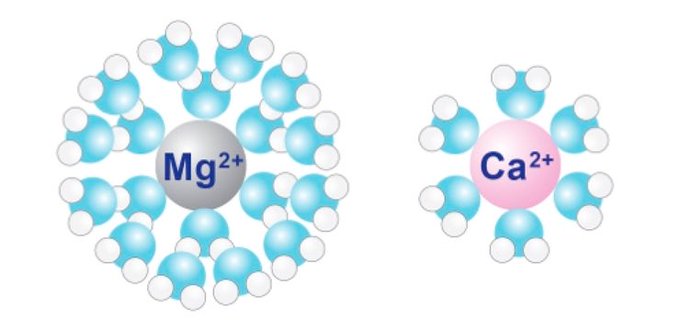
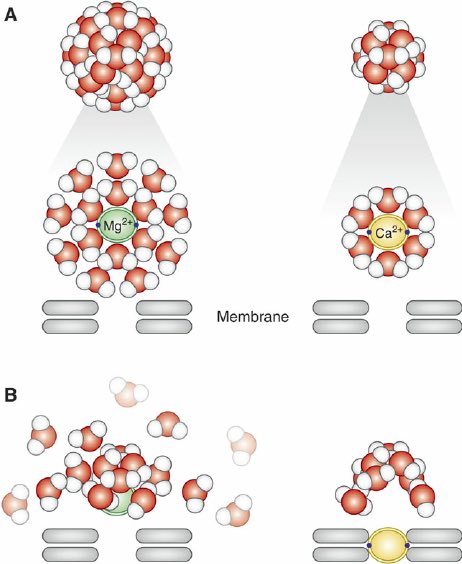
Water is a molecular dipole, the oxygen side holds more negative electrons, while the two hydrogens are more positive This means a "hydration shell" of water molecules will cluster around charged ions dissolved in water Magnesium forms a larger hydration shell than calcium
Calcium's small hydration shell allows it to diffuse quickly Because calcium and magnesium have the same +2 charge, calcium binding sites in proteins are occupied by magnesium at rest Sudden calcium influx displaces it, activating various proteins and stimulating the cell
This is also what allows magnesium to act as a calcium channel blocker It binds calcium channels, such as the excitatory NMDA glutamate receptors in neurons, but its hydration shell is too large to pass through This gives it an inhibitory effect on muscle, neuron firing, etc
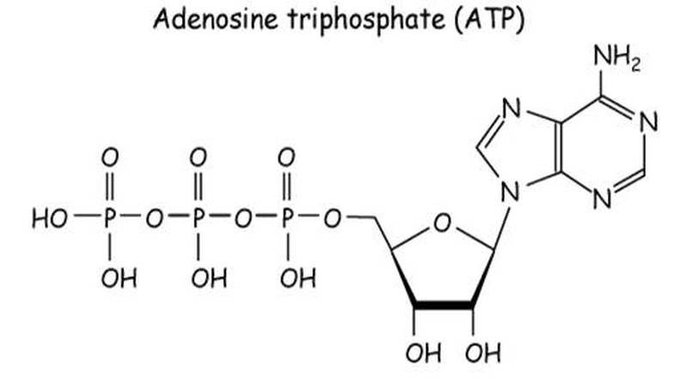
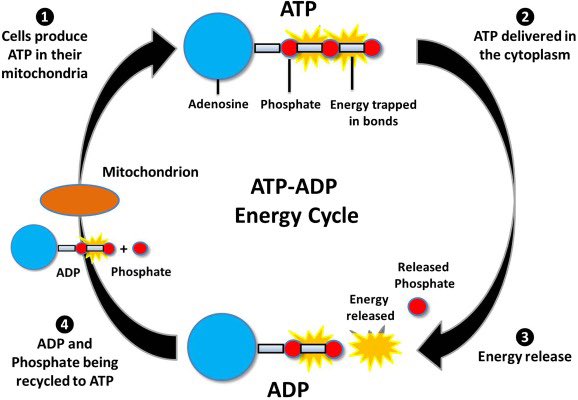
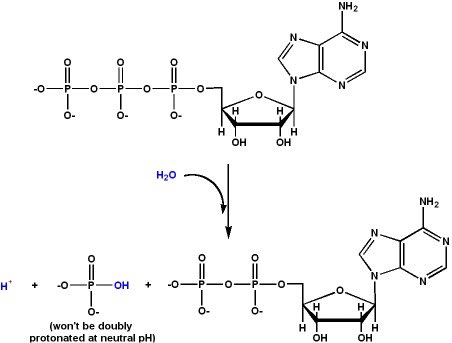
Another important role magnesium plays is its interaction with ATP ATP serves several purposes in living cells It contains a tail made of three phosphate groups, and directly activates many protein by attaching phosphate groups to them It also acts as a marker of energy status
This is because the level of ATP is directly coupled to the charge separation that mitochondria use to generate energy Normally in solution, ATP will rapidly degrade into ADP by losing phosphate groups The cell maintains a far-from-entropy state by keeping a high ATP/ADP ratio
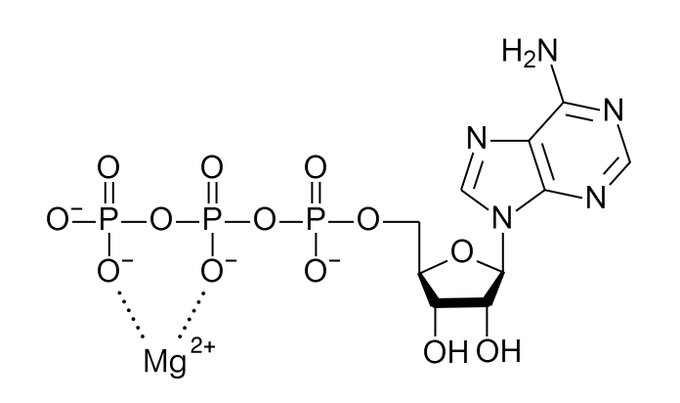
Magnesium slows down the natural breakdown of ATP into ADP by stabilizing its phosphate chain Magnesium is ideal for this purpose because its high charge/size ratio allows it to form a tight bond with the phosphate groups Replacing it with other ions tends to destabilize ATP
Magnesium also acts as a direct cofactor by speeding up various reaction in cells Some estimates suggest that more than 40% of all enzymes in the body require magnesium to function Often this stems from magnesium's ability to bind nearby water rather than magnesium itself
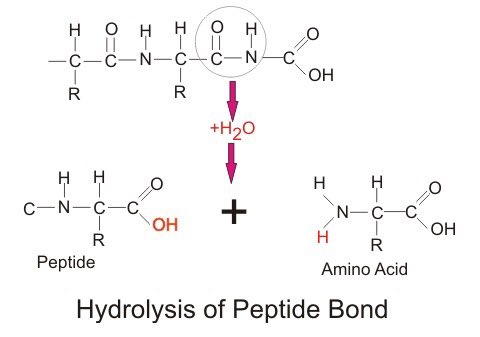
A good example of this is hydrolysis reactions Hydrolysis refers to a type of bond-breaking reaction that uses water as a cofactor Usually this involves splitting water into -OH and +H and attaching each half to one of the resulting byproducts, such as in breaking peptide bonds
Another reason magnesium works closely with ATP is that breaking the phosphate bond in ATP also involves hydrolysis This means proteins that are phosphorylated (activated) by ATP usually have a magnesium binding site that provides water Magnesium is necessary to burn ATP
Other factors in biology including GTP, vitamin B1, glucose, and creatine have phosphate forms that require magnesium to stabilize or use Magnesium is also a mild Lewis acid and can speed up reactions that require acidity DNA and other proteins even coil around magnesium ions
HOW TO SUPPLEMENT MAGNESIUM Magnesium is one of the most accessible, affordable, and well-tolerated supplements on the market I think just about everyone could benefit from using it First, let's talk about two concepts: "magnesium burn rate" and "magnesium saturation"
The "burn rate" for magnesium refers to how much the body uses in a given period of time Factors like age, stress, disease, aging, and physical activity can all affect your daily magnesium requirement This concept applies to other nutrients like potassium and B-vitamins as well
Magnesium saturation occurs when the magnesium supply is enough for the body to fulfill its requirements, with some extra Most people don't realize that bone doesn't just serve as a reservoir for calcium It's estimated that more than 10 grams of magnesium can be stored in bone!
When magnesium burn rate is high, and magnesium intake is low, the storage levels of magnesium can be depleted over time This is compounded by the fact that modern agriculture has led to most fruits/vegetables being significantly depleted of magnesium due to low soil levels
Magnesium storage also means that blood tests like serum magnesium are not able to detect deficiency Patients often end up in the ICU before they're diagnosed with deficient magnesium https://www.ncbi.nlm.nih.gov/p... RBC magnesium is a more accurate test that looks at cellular levels
Some estimates suggest as much as 90% of the population is magnesium deficient Often this is "sub-clinical" deficiency that could take years to cause problems, but severe illness will increase its severity This is why I'm so passionate about supplementing magnesium
So how do we reverse deficiency and get to a point of magnesium saturation? There are three main ways to get more magnesium: - eat magnesium-rich foods - use a magnesium supplement - apply magnesium topically Let's briefly look at all three options
Magnesium-rich foods include: - hemp seeds - sprouted brown rice or oats - spinach/kale/chard/beet greens - pumpkin/chia/flax seeds - dark chocolate (make sure it's low in heavy metals) - avocados - bananas - meats like wild game/turkey/fish - potatoes and other root vegetables
When it comes to magnesium supplements there are various forms to choose from The most important thing to consider is how well each form absorbs Well-absorbed forms: magnesium glycinate, taurate, lysinate, malate Poorly absorbed forms: magnesium citrate, oxide, hydroxide
The magnesium content of each form also varies, since whatever carrier the magnesium ions are bound to makes up a % of the weight The percentage of "elemental" magnesium lets us calculate the magnesium it provides Total weight in mg x decimal percentage = magnesium content
So what's the best way to dose magnesium? There are many protocols available but my personal approach is to use as much as is tolerated orally Too much magnesium in a serving, especially of poorly absorbed forms, can induce a laxative effect and this should generally be avoided
The "bowel flush" from magnesium is actually due to its ability to hold water once again Magnesium absorption channels occur in the upper intestine, so if unabsorbed magnesium makes it to the colon it prevents water removal Other nutrients like calcium can work the same way
This means the optimal way to dose magnesium is smaller doses spread throughout the day (ie 100-300mg) The bowel flush can be useful to relieve constipation, but it's best to avoid it long term Taking magnesium with food/water also seems to improve its absorption
My three favorite forms are: - magnesium glycinate - magnesium taurate - magnesium chloride The first two can be purchased as powder or pre-filled capsules, and taken throughout the day/before bed Magnesium chloride can be dissolved in water and sipped throughout the day
I highly suggest buying gel caps and bulk magnesium powder and making your own capsules to save money If you find filling capsules tedious, you can use a "capsule machine" or filling tray If using food grade magnesium chloride, I would simply start with ~1/4 tsp in water
This brings us to topical magnesium There are two forms of magnesium that are used topically: magnesium chloride and epsom salt (magnesium sulfate) Either form can be dissolved in water and applied topically using a spray bottle, bath, or foot soak
Magnesium chloride spray recipe: - Buy a glass spray bottle - Measure out 1-2 cups of water - Measure 10-25% of the volume of the water as magnesium chloride, add to water until it dissolves - Add a few drops of essential oil for scent if desired This means 25% magnesium solution is 1 cup water + 1/4 cup magnesium It may be necessary to lightly warm the water to fully dissolve the magnesium Magnesium sulfate can be used instead if you find chloride itchy/irritating